Authors: Alicia Hernandez-Mora, Olivier Duboc, Else K. Bünemann, Kari Ylivainio, Enzo Lombi, Sarah Symanczik, Dietmar Horn, Antonio Delgado, Nadine Abu Zahra, Lucia Zuin, Casey L. Doolette, Herbert Eigner, Jakob Santner
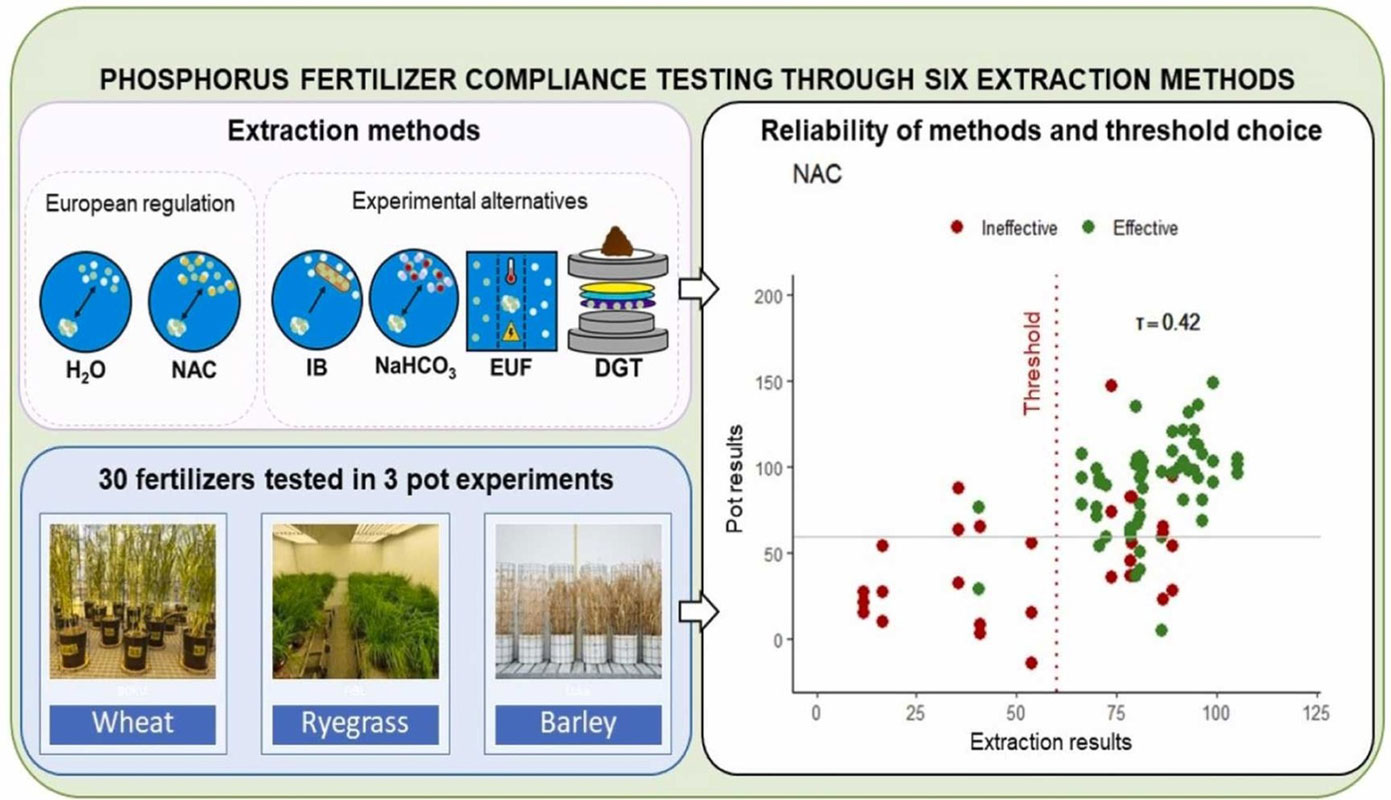
Journal: Environmental Technology & Innovation Abstract: Phosphorus (P) recycling for fertilizer production addresses the dependency on phosphate rock and mitigates P losses to the environment. However, predicting plant-available P in recycled fertilizers is challenging due to their diverse chemical composition. This study aimed at identifying the most suitable P extraction method for fertilizer compliance testing, considering their correlation with actual fertilization efficiency, as well as their simplicity, throughput, recognition and cost. Studies on fertilizer P compliance testing often lack recommendations on minimum P extractability threshold values. Here, thresholds are calculated based on actual fertilization efficiency of a large, chemically diverse set of recycled P fertilizers, many of which are already marketed. Thirty recycled P fertilizers were extracted with H2O, neutral ammonium citrate (NAC), electro-ultrafiltration (EUF), ferrihydrite-filled membranes (iron bag; IB), sodium bicarbonate (NaHCO3) and diffusive gradient in thin films (DGT). The mineral replacement value (MRV) of the fertilizer set was previously evaluated in three pot experiments at a fertilization rate of 50 mg kg-1 soil. MRV correlations with the extractions methods showed similar results for all besides H2O, which cannot be a reliable indicator for P availability. Fertilizers were classified as efficient or inefficient based on their MRV exceeding or falling below 60% of the triple superphosphate reference value. The minimum P extractability threshold value (MPETV) for each method was based on the efficiency classification and it minimized the number of misclassified fertilizers. NAC, with a 60% extractable minimum P threshold value, was the most adequate method for compliance testing, despite its overestimation of iron phosphate availability.
The paper is open access and available via the following link:
https://doi.org/10.1016/j.eti.2024.103913