Effect of LOHC on HDPE and POK under different temperature conditions
VIDEO
coming soon...
Introduction
The future energy carrier and significant potential energy source is hydrogen. Due to its low volumetric density, hydrogen is difficult to store. On the other hand, a certain type of hydrogen in the form of a liquid can be used to store significant amounts of hydrogen in thermoplastic tanks under ambient conditions. However, keeping hydrogen in this form for a longer period of time in polymer tanks has an impact on the liner's physical and chemical characteristics. High-density polyethylene (HDPE), polyamides (PA6, PA12), and a few other thermoplastics were already being used in gasoline tank applications in the present car industry. However, prolonged contact with fuels causes hydrocarbons to seep into the polymers, losing its mechanical characteristics as a result. Therefore, a substitute material is sought specifically for applications in hydrogen fuel tanks. In order to measure the mass uptake in these materials, the two semi-crystalline thermoplastics high-density polyethylene (HDPE) and polyketone (POK) were compared and exposed to a chosen liquid organic hydrogen carrier (LOHC) at 25 °C and 60 °C for 500 hours.
Materials and Methods

Results & Discussions
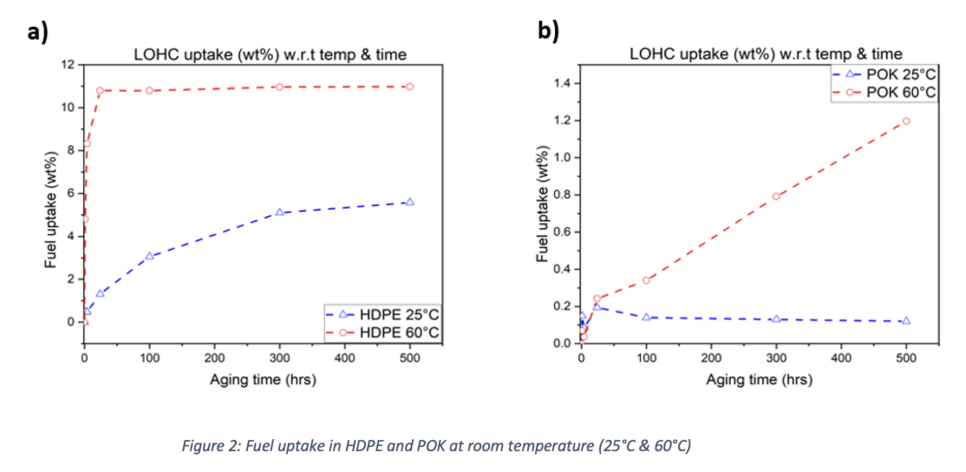
HDPE attained a plateau at 60 °C after 500 hours of exposure in LOHC. Additionally, it is observed that as the temperature rises, the rate of HDPE absorption rises as well because at high temperatures, polymer chains are more mobile, increasing the free volume of thermoplastics and facilitating easier molecular diffusion [33]. Because atoms are more closely packed in amorphous regions at low temperatures, the absorption rate of chemical fluids into polymers is much lower at 25 °C.
On the other hand, when compared to between 100 and 500 hours, aged samples of POK showed a considerably smaller rise in weight with rising temperature. For both 25 and 60°C, there was an initial decrease in mass at the start of the exposure time. Although there was no degradation during these testing, this drop may be related to the volatilities of leftover water or organic compounds that may evaporate with time. Gas chromatography was used to make sure the substance had not been dis-solved in LOHC, but neither olefins nor carbon monoxide (CO) were detected. Additionally, a centrifuge machine (Thermo Electron's Heraeus Labofuge 300) was employed. Small glass tubes were placed within the centrifugal unit and rotated for 300 seconds at 2000 rpm.